The Agricultural and horticultural products regulatory review is open for comment until 8 September 2024. The review will focus on the approval path for new products, aiming to ensure that the ‘risks of products are known and appropriately managed, including to human health, trade, animal welfare, agricultural security, and the environment.’
It looks like the time frame is too short, and the terms of reference plays directly into the hands of the importing drug and agricultural companies, while downplaying more complex considerations relating to risk and benefit, trust and safety for our growers, farmers, veterinarians and the New Zealand public.
What is in scope? Agricultural and horticultural products that are currently regulated under the Agricultural Compounds and Veterinary Medicines Act 1997 (ACVM Act).
Officials will focus on the assessment and approval process (approval path); the reassessment processes (including the thresholds for triggering reassessments); any overlap with the Hazardous Substances and New Organisms (HSNO) Act 1996; as well as links and overlaps with offshore regulatory agencies.
These issues are commensurately familiar to drug and agricultural compound importers who conventionally apply for approvals so that their products can be marketed and sold in New Zealand.
The review ‘seeks to understand’ public interest matters, including market failures, risk thresholds and the basis for government intervention; the costs and benefits of the regulation and how well the regulations are working. But can this be even vaguely achieved?
First of all, the basics:
What is an agricultural compound?
The following classes cover the majority of the term “agricultural compound”:
- veterinary medicines – substances used for animals, including companion animals
- agricultural chemicals – substances used for plants, including herbicides, fungicides, insecticides, plant growth regulators, surfactants, and adjuvants
- vertebrate toxic agents – substances that kill or limit the viability of animals, such as possums, rodents, and other unwanted mammals
- fertilisers, plant biostimulants, and soil conditioners
- pet food and animal feed – including dietary supplements.
When products such as disinfectants and cleaners are used to maintain hygienic conditions for animals and plants they also come under this category. Absorbable sutures or microchips can be considered agricultural compounds if they have a non-removable chemical in them.
REVIEW TIME PERIOD
This regulatory review time period is far too short for many of the smaller and medium sized businesses to appropriately comment. I’m hoping all our food producing industry sector representatives and veterinary staff operating in small businesses will have time to respond. The time period, however, suits well-resourced corporates across Big Pharma, Big Ag and Big Chem who liaise intensively with MPI when seeking approval status to import their products.
THE TERMS OF REFERENCE – & NATIONAL KNOWLEDGE ‘FAILS’
The Terms of Reference (PDF on this page) state that:
The review will aim to achieve this in part through:
- looking at the individual regulatory systems as a whole from the viewpoint of those trying to seek approval through them;
- understanding what is the problem being addressed by the regulation and whether the regulatory systems are achieving their stated purpose within the context of this review;
- grounding the review in economic analysis of the market and regulatory interventions, including consideration of the underpinning market failures and the costs and benefits of regulation;
- benchmarking our approval path against comparable international regulators and international best practice; and
- considering how the overlap and interface between the HSNO and ACVM regulatory systems is managed by government agencies.
Those ‘trying to seek approval’ are overwhelmingly represented by the importing companies who are the ‘applicant’ for assessments and reassessments.
What is the problem being addressed by the regulation? This is where it gets muddy, because what the importers claim may be different from New Zealand’s farmer and grower representatives and individual growers and farmers.
WHO SUPPLIES THE SCIENCE TO SECURE A PRODUCT APPROVAL?
Non-MPI officials undertaking this review face extraordinary barriers to securing a broader appreciation of ‘public interest matters, including market failures, risk thresholds and the basis for government intervention’ if they wish to understand the broader risk landscape beyond the applicant’s perspective.
Regulatory science is political, cultural and social – a regulators concept of objectivity is predominantly a function of the powerful forces that surround them. Regulatory science also lags behind the scientific literature.
By convention, it is the importers (the ‘applicant’) seeking approval of a compound. Regulators globally depend on the applicants to provide the bulk of data supporting a product assessment or reassessment. The importer will also frame the costs and benefits of their formulation. The effect is a global regulatory round-a-bout of company-driven information with agencies keeping contradictory evidence at arm’s length. This explains why New Zealand’s Environmental Protection Authority could state that there was no evidence to reassess glyphosate, particularly when ‘considered alongside the findings of other international regulators.’
Funding for drug and agricultural compounds approvals and reassessments are derived from industry levies. This dependence can result in a decline in evidentiary standards, and a pivot to seeing problems from the perspective of the company they are charged with regulating.
There is no scientist or research group in New Zealand who is funded to look at these issues and consider the broader scientific literature. There doesn’t seem to be a budget for the ACVM Group within the Ministry for Primary Industries (MPI), or the Environmental Protection Authority, to fund our research institutions to independently assess ‘market failure’ or risk thresholds in New Zealand.
NO SCIENTIFIC FREEDOM TO VERIFY CLAIMS OF TECH PRODUCER/IMPORTERS
The risks will only be known, insofar as the data that the veterinary drug and agricultural compound importing industries of concern collect for regulatory approvals, are prepared to declare. The risks will only be understood by regulatory agencies, as far as their protocols and guidelines permit them.
This is not a problem for generic drugs and compounds with a long history of safe use. But new and novel compounds which include biologic drugs prone to contamination, or under-regulated technologies, where claims around safety and efficacy are based on limited animal testing data and antibody response can produce unexpected and off-target harms which persist in human bodies and the environment.
This is not necessary in well understood technologies, but new technologies are yet to be fully understood.
Important research can shed light on under-recognised hazards from approved compounds. This can include assessing mixture synergies, body burdens by chemical class, and non-linear hormone responses.
But there are no research funding pathways for this long-term basic and applied research work.
This is how farmers, growers and the general product can trust existing and new technologies. We may trust (corporate claims) but claims with a financial conflict of interest must be verified independently.
The purpose of the ACVM Act is to manage risks – but when there is no scoping, no feedback loops, no freedom of scientific inquiry, the ball is completely in the drug and agricultural chemical companies’ court.
The gap creates a greater burden for businesses. The only way everyone from veterinarians to food-producers and export industries can navigate corporate claims, is by surveying everything from regulatory decisions, to the scientific literature, to documents released in court trials, and by understanding which countries are quick to turn back tainted product or showing harm.
While there is no scientific freedom to assess the claims of drug and agricultural importers, the ACVM Act, is largely hopeful and hypothetical. Assessments of the scientific literature, reduction of dependence on old modelling standards and increased surveillance and reporting mechanisms from primary producers, veterinarians and scientists is essential. Funding and political freedom for biomonitoring, at arm’s length from government agencies, is also critical.

When such issues are out of scope, it is easy for the importers to glide over the problems, and assure David Seymour, Penny Simmonds and Andrew Hoggard that everything will benefit the economy.
When the agrichemical and biotech industry wails about the economic necessity, we simply do not have the scientists who can trust but verify. New Zealand’s Attorney General, and Minister for Science, Innovation and Technology Judith Collins, recently denied that there was an absence in New Zealand’s $1.2 billion science budget of funding for research into risk from toxic chemicals and genetically engineered organisms, and that this funding deficit would limit New Zealand’s capacity to assess risk over the longer term.
Ask risks are identified, we don’t have the Environmental Protection Authority contracting universities and CRIs to conduct global reviews to understand how farmers and grower’s practices alter when these chemicals were banned, to identify nutritional or integrated pest management strategies (IPM) put in place in foreign jurisdictions to support a transition away from a banned product, and to identify less-toxic substitute products to ensure productivity and profitability is not impacted.
THE DOUBLE BIND OF OUR FOOD EXPORTERS
If this agricultural and horticultural products regulatory review were undertaken in such a way as to meaningfully prevent or manage risks the review, officials need to dip their toes in a broader range of complex and overlapping issues far beyond the struggles corporate applicants have with New Zealand’s new product agriculture and horticulture approval process.
This includes recognising that trust in regulatory science will only work if bureaucrats have the moral courage to ally New Zealand with the safest regulatory jurisdictions; the political will to monitor ‘politically controversial’ compounds; and courage to consider ordinary toxic synergies that are key to the success of a retail formulation.
Regulatory agencies rarely deviate from company claims relating to risk and economic benefit. Unfortunately, they don’t have the remit and resourcing to look too deeply. Their guidelines can lock in quaint modelling concepts, write out epidemiological evidence, ignore childhood risk and dismiss the toxicity of the full formulation that is central to the economic success of a compound.
Without uncomfortable feedback loops the evidentiary basis can fall into disrepute and public trust can decline. Export industries can be stuck in an uncomfortable, and face added costs in safety testing.
The evidence suggests that domestic food export industries have to ignore official statements of safety (based around ACVM and HSNO act regulations). Their most important issue is not getting caught by more rigorous offshore regimes with an eagle eye on residue limit exceedances for drugs and agricultural compounds.
Conventional regulatory ‘economic calculations’ directly protect the claims of drug and chemical importers, but make it impossible for our food producers and exporters to judge global best practice.
Alarmingly, if it’s politically controversial, New Zealand’s ironically named Food Safety steers clear of testing. Food Safety might have had a strategy refresh, but the Director-General of Food Safety is a career bureaucrat who lacks a background in toxicology, public health, and/or agribusiness. Food Safety’s swanky new strategy with lots of Māori words in it mentions ‘safety’ 96 times, but doesn’t mention ‘pesticides’ or ‘genetically modified foods’ or ‘toxicity’ once. Keeping up to date with monitoring toxic compounds in the diet is not as important as monitoring their performance.
I suspect plenty of Māori understand that all the manaaki and whanaungatanga in the world means nothing if the agency ignores toxic chemicals in food.
It’s a blank slate for drug and chemical importers, because there’s no Food Safety language around toxic pesticides, genetically modified foods and the accumulated exposures associated with these technologies.
New Zealand food exporters have to very carefully consider each country that they are importing into, and ensure that chemical residues in their export product sits under those levels.
‘Food Safety’ prioritise harmonising with Codex residue levels, when Codex residue levels (such as glyphosate) can be much higher than European residue levels. As an example, the last serious Codex glyphosate evaluation occurred just as glyphosate companies were introducing glyphosate onto food crops as a pre-harvest desiccant. The biggest global producer, China, pragmatically controlled the 2019 evaluation.
There are stark differences. Europe won’t be spraying diquat or glyphosate on cereal crops as a pre-harvest desiccant because diquat is banned and glyphosate is severely restricted. But in New Zealand diquat can be sprayed on green peas, wheat, barley and more, and glyphosate can be sprayed on our wheat and porridge oats.
New Zealand might have maximum residue levels for diquat (2 mg/kg) and glyphosate (0.1 mg/kg) but New Zealand’s ‘Food Safety’ authority doesn’t want to test for them. These toxic chemicals are being ignored in the 2024 New Zealand Total Diet Study (Infants and Toddlers). They haven’t been tested in the past, either.
Nor do Food Safety monitor foods for contamination from genetically modified food ingredients.
In a 2023 consultation, most people who submitted, wanted ‘Food Safety’ to test for glyphosate. In the past, glyphosate levels in wheat have been exceeded. But Food Safety chose to ignore an obligation to check residue levels by dribbling on about the International Agency for Research on Cancer while conveniently ignoring New Zealand’s failure to risk assess glyphosate for thirty years and their failure to regularly monitor glyphosate in crops that are traditionally sprayed just before harvest. When it comes to levels on animal feed, Europe has some knowledge. We don’t do that stuff.
SCIENTISTS AREN’T FUNDED TO BE CURIOUS ABOUT TOXIC CHEMICALS
New Zealand has a basic science deficit and our testing laboratories are privatised. The Crown Research Institutes (CRIs) have to run at a profit. Researchers who want to understand if a formulation is more toxic than a declared active ingredient struggle to get access to the product, due to commercial in confidence reasons. Then of course they have to get funding for testing in our privatised labs.
The government doesn’t have a language for long-term public good research that might inform growers and researchers, especially when it comes to long-term stewardship of pasture, and our arable and horticultural regions.
Running at a profit for New Zealand public science institutions means lots of public-private partnerships where the job is product development and the end game is release into the market of a patented product. Sometimes CRIs will link up with agricultural industry groups, but industry tends to be focused on worthy short-term problems. Overlapping issues that cut across different sector, that are long-term are much less likely to get addressed.
To understand the lay of the land, David Seymour, Andrew Hoggard and Penny Simmonds could ask questions to understand if existing approved veterinary drugs and agricultural compounds are well stewarded.
They could request reports of the top 50 veterinary drugs used in New Zealand; and the top 100 agricultural compounds by quantity of tonnage applied directly to food crops. They could then ask officials to review whether these chemicals were tested in the 2024 New Zealand Total Diet Study (Infants and Toddlers).
They could request a report assessing which pesticides in use in New Zealand are banned in other countries. Which countries have heavy regulations preventing applications of certain pesticides on food crops.
They could survey veterinarians to assess which drugs they consider firstly, essential in current practice (this includes generics); and secondly, most useful that they have identified in the scientific literature but that they struggle to gain access to. They could also ask veterinarians if existing regulations on some veterinary inputs are too onerous for their existing safety profile, or not onerous enough. They could review chemicals biomonitoring in other countries and contrast this with domestic approaches.
The ‘safety and efficacy’ of the approvals process is wrapped around what we know and what we don’t know.
NEW TECH – E.G. BIOLOGIC DRUGS
The policy and legislation has to not only be fit for run-of-the-mill applications but for new technologies which, with only company data, may over time, present greater risks. But regulatory protocols ensure risks are framed by the importer – this is the way all regulatory agencies globally operate.
Obligations and guidelines around new generation biologic drugs have been remarkably unstudied. The ACVMA Act states:

It is August and the disgraced Therapeutic Products Act 2023 (TP Act) is yet to be repealed. The focus for most people concerned the very real potential of this Act to produce barriers to trade in nutritional compounds in human diets, that have a very long history of safety. Nutritional compounds don’t promise the same blockbuster incomes as chemical and biologic drugs, as it is a rare occasion when a nutrient formulation is patented.
The big gap – the gap that the Physicians and Scientists for Global Responsibility focussed on, concerned persistent uncertainties around new biologic drugs, where all too often regulatory guidelines are based on the studies done by the company and the parameters (or not) set by institutions who struggle to act independently from the companies that finance them.
Biologic drugs require very strict regulation and oversight. But the Therapeutic Products Act did not take steps to account for the risks and uncertainties which are registered in the scientific literature, problems with safety and efficacy, which the gene therapies used in COVID-19 brought to light.
Biologic drugs as veterinary medicines are unlikely to be any more rigorously reviewed for safety.
DRUG DEVELOPMENT – CONFLICTS OF INTEREST IN OUR AG SECTOR?
Envisaged new technologies including gene therapy and methane inhibiting technologies, would be regulated through the ACVM Act. mRNA so-called vaccines would come under this review. They might be gene therapies but they are not categorised as genetically modified organisms through regulatory decision-making.
There’s an interesting twist. MPI is a fifty-percent shareholder, together with ANZCO Foods, Fonterra, Rabobank New Zealand, Ravensdown, Silver Fern Farms, and Synlait – of a ‘climate mitigation’ startup called Agrizero. These dominant companies that farmers buy from (Ravensdown) and sell to, ANZCO Foods, Fonterra and Synlait, are hoping to benefit from products that would be regulated under this legislation, and that could well be ‘mandated’ by the government.
Many of these companies are dominant exporters, so theoretically they might inform the government of how to make New Zealand regulator safer to ensure that consumer trust doesn’t backslide. But as new partners in a biotech startup they might be advised by sharp-eyed lawyers to keep the approvals process company-friendly.
I’d be regarding these companies with a sceptical eye. Are they looking at long term trust in premium markets or being advised on how not to upset the regulatory apple-cart?
SCIENTIFIC FREEDOM EVISCERATED BY AN ABSENCE OF RESEARCH FUNDING
We can see that the regulatory agencies don’t have the budget to explore new biotech technologies further. I suspect there is not the scientific freedom in New Zealand for a laboratory to run a mixed methods research programme and understand the suite of risks that are presented with biologic tech. For example, to take a New Zealand made or imported vaccine product based on novel technology and designed to supress a transmissible virus, and critically assess the efficacy against the changing strain profile over time and vaccine failure against subsequent reinfections. Conduct a literature search to assess the likelihood of a changing strain profile. It’s very easy for claims around efficacy to downplay important issues – such as the real effect on a transmissible agent if a drug is only 50% effective in in vivo experimentation.
Regulatory claims around efficacy during COVID-19 were based on selective attribution, not on reviews of the scientific literature which would demand scholars to consider the weight of evidence and judge risk in a more nuanced fashion. Uncertainties, gaps and inconsistencies in gene therapy trial data, despite being raised by regulators, can then be downplayed and ignored.
If there was scientific freedom, scientists could run invitro tests to understand the toxicity and persistence of all components in a drug formulation. Run metabolomic tests to understand for example, whether a biologic drug induces type I interferon (IFN-I) responses, setting the stage for inflammation and autoimmune problems. Conduct biodistribution tests to assess whether an injection which is presumed to remain in the bloodstream, moves elsewhere in the body. Assess whether the compound has impacted the reproductive tract in both unborn livestock and in the first year after treatment.
If there was scientific freedom, our local scientists could trust but verify, that RNA in gene therapies does not reverse transcribe – that the mRNA vaccines are not incorporated in the genome. Understand that as mRNA vaccines encode for a single antigen in most cases, the risk of immune escape is heightened. Or worse, the tech might promote antibody or immune-dependent enhancement. As different batches are marketed over time, domestic laboratories could test batches for contamination. Gene therapies are biological drugs prone to contamination.
For new drugs including those intended to alter methane output, ensure drugs are tested intergenerationally in live animals to understand the impact on nutrient absorption, uptake and microbiome, gastric fitness, productivity and reproductive health. Release the underlying data. The frequency of the drug over time can be tested against all these parameters, and not by the institutions which would also hold patents and receive royalties from that drug.
Each trial must be publicly registered, so as to understand the balance of success and failure.
There’s a lot of work that is ignored and dismissed, but essential to sustain long-term trust, by growers, farmers, Kiwis, and export markets.
CONFIDENTIALITY FAVOURS THE CORPORATION
Confidentiality persistently orients to favouring public sector and company secrecy. The Industry Liaison Group and Advisory Council meet three times a year. However, specific information about individual members of the ACVM Group is not declared. There’s quite a bit of non-disclosure.
MPI officials responsible for approvals and reassessments are undeclared, yet importers dealing with MPI will know exactly who these actors are, as there will be approvals-related crosstalk between industry and MPI. Remember, the data is supplied by industry, and very old data can lock in a product as safe for a very long time. There’s no ‘cream’ in the budget for officials to consider risk outside industry-supplied data.
Drug companies don’t like independent scientists gaining access to new-to-market drugs to undertake research that might raise questions about safety and impact return on investment. The companies are helped by the laws which include the ACVM Act.
Without up-to-date regulation that reflects changing knowledges and new challenges, it is evident that the economics favour the drug and agricultural compound importer.
GROUNDING THE REVIEW IN ECONOMIC ANALYSIS
Farmers, growers and veterinarians make judgements over the long term, not on a short-term basis.
Long-term considerations for farmers and growers are based on balancing profitability with input costs from three to twenty years. Veterinarians must consider the productivity, health and fitness of livestock both for the animal in front of them and the fitness of their progeny. They don’t want unexpected cancers and autoimmune conditions popping up in animals with no prior history of that disease.
The Terms of Reference are:
- grounding the review in economic analysis of the market and regulatory interventions, including consideration of the underpinning market failures and the costs and benefits of regulation;
Market failure includes failing stock health, declining productivity and reproduction. It includes soil that is contaminated by multiple complex chemicals, applied over time that bind nutrients and reduce uptake.
Importers with their eye on sales won’t review the incidence and potential of drug (including antimicrobial) resistance that might mean that a drug might only be meaningfully effective for a short time. They won’t consider off-target effects from outdoor gene editing in agriculture.
They won’t assess dietary adjustments that might contradict a claimed need for a commercial product.
But these problems (and some solutions) happen over the longer term, and regulatory parameters have so many holes, you can strain pasta through them.
Most of what I have described in this article, will most likely be out of scope.
The only way the New Zealand consumer, or agricultural product intended for the domestic market can benefit, is if our food exporters adopt a defensive practice designed to prevent products being rejected at international ports for exceeding local residue levels of an agricultural compound or drug.
This defensive second-order protection route is also the only way domestic consumers are protected, unless they elect to purchase much lower residue organic product.
We’ve seen this occur in honey. Japan rejected New Zealand honey as it was tainted with glyphosate. MPI tests a few years ago showed that around 20% of domestic honey continues to be contaminated.
But the top marks for current the export product indicates that exporters are maintaining an eagle eye on all exports. With the NZEPA disinclined to restrict spray regimes, even though testing for glyphosate is expensive, our honey industry has simply had to absorb these costs.
- benchmarking our approval path against comparable international regulators and international best practice;
This is where it is interesting politically. To generalise, Europe tends to have more scientists looking at more issues, while the USA tends to focus on getting tech to market. Which country might have a stricter regulatory regime? Who should we benchmark against?
We’re observing industries approaching Australian and New Zealand regulatory agencies to secure approvals for chemicals and biotechnologies, when the only approvals to date are in the USA and Canada – but not Europe or Asia. This was evident when the 600 gm/L glyphosate was approved. This may suggest that in the OECD, New Zealand regulatory authorities are the next weakest link in the chain after North America.
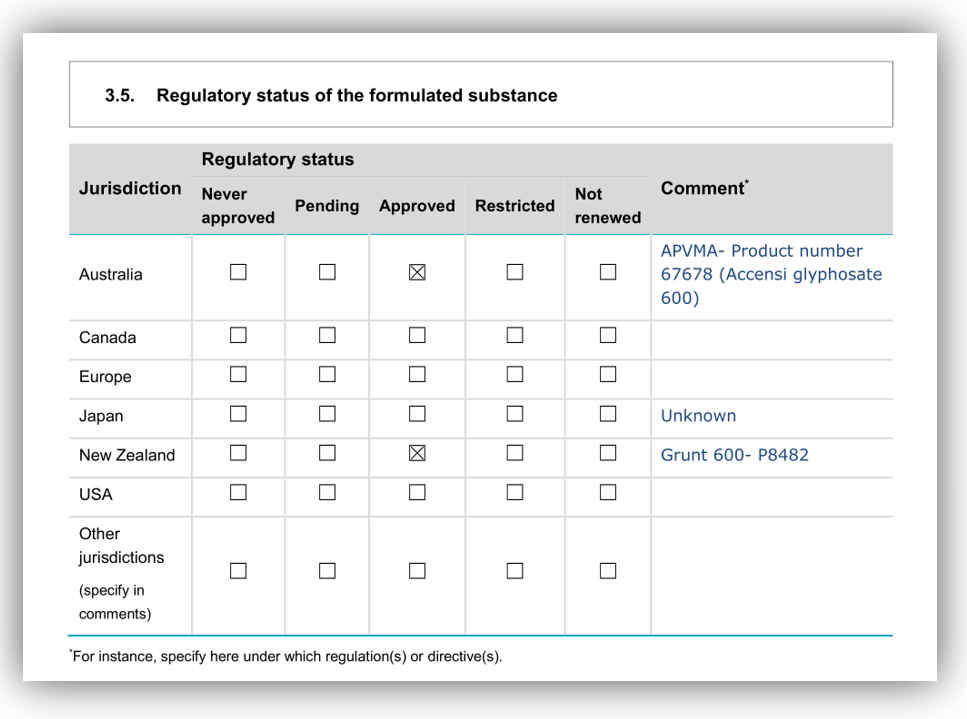
Of course, then there are the chemicals that are banned or severely regulated in Europe but still available here. (Note, when pesticides not used specifically for agricultural purposes will not come under the ACVM Act.) A selection of such chemicals banned in Europe but not here include:
Mancozeb, paraquat, chlorpyrifos, methyl bromide, hydrogen cyanimide, hexazinone, terbacil, atrazine, haloxyfop-P, thiram, methomyl, carbaryl, diazinon, dichlorvos, 1080, brodifacoum flamprop-M-isopropyl, isoproturon, fenitrothion, Iprodione, Methamidophos.
The charity I am a member of, Physicians and Scientists for Global Responsibility New Zealand has spent many years proposing that more scientific research in these areas is undertaken to reflect the gaps in knowledge and the changing landscape (such as here and here and here and here).
But when we respond to submission processes, our discussions, which traverse social issues such as a need for more expertise, and scientific and political evidence demonstrating why guidelines and processes are too narrow and outdated, and hence fail to be consistent with the purposes of the legislation, – we’re soundly ignored.
It’s unfortunately apparent that this current review is also time poor and under-resourced. And, without paying sufficient attention to actors outside the corporate and patent-seeking industries, people who don’t have the most familiarity with the approval process – simply because they are not the importers that engage intensively with MPI – the review is unlikely to achieve its aims.
However, unfortunately, there are insufficient ‘experts’ out there who don’t have economic skin in the game, and who aren’t funded directly by industry who might be seen as authoritative.
It’s very clear that when a subject is politically controversial and contradicts Ministry positions, scientists terrified of losing funding status, or worse, being let go in the next round of job cuts, won’t speak up, even if they are subject-matter experts.
Scientific freedom has been declining for 30 years. This has had a direct political effect on how we approve and govern technologies.
Thank you Mary for another well researched and informative article – your efforts are much appreciated!